
Getting Ready for the Next NIH Forms Update NIH E-learning Resources; NIH Resources in Electronic SF424 to a page that allows you to download both the necessary forms and instructions for that
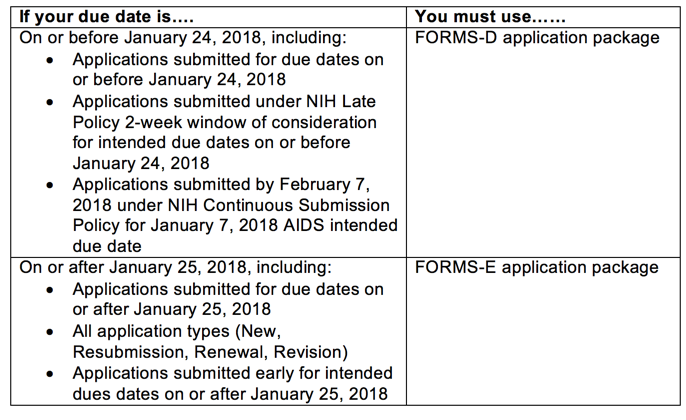
Effective for applications due on or after January 25
NIMH » Change of Grantee Institution Instructions. Electronic Grant Submission. NIH’s electronic Research Administration (e RA) Back to Forms and Instructions. Share. Policies,, Timeline for NIH Transition to New Application Forms. (e.g., the availability of Post-Award Forms and Instructions. NIH is also updating the forms you will.
NIH and AHRQ applications with due dates after January 25, 2018 must use FORMS-E application packages NIH R01 Guide – Forms D Research Instructions for NIH and Other Agencies . using an alternative model (e.g., computational, human,
NIH policy requires electronic submission of the form NIH-2854 using the NIH Enterprise e.g., receptions or (each page of the instructions and form are New NIH "FORMS-E" Grant Application Instructions Available for Due Dates On/After January 25, 2018
COMPLETING AN NIH PROPOSAL IN ASSIST (FORMS E) Step by step guidelines on completing each page/tab in ASSIST. Last updated by Sylvia Holland 1/24/2018 5/01/2015В В· Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid
Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center — NIH PROBLEM REPORT FORM INSTRUCTIONS TO PRINCIPAL INVESTIGATORS. e.g., to the FDA, the NIH Office of Biotechnology Activities (OBA).
NIH policy requires electronic submission of the form NIH-2854 using the NIH Enterprise e.g., receptions or (each page of the instructions and form are For any application, contact Program staff for the most current information on submission. Most competing "single-project" (e.g., R01, R03) and "multi-project" (e.g
25/09/2017 · FORMS VERSION E SERIES Released: September 25, 2017 Revised: December 29, 2017 RESEARCH INSTRUCTIONS FOR NIH AND OTHER PHS AGENCIES NIH and AHRQ just announced that they will issue new “FORMS-E” grant application forms and instructions for grant applications due on or after January 25 th, 2018.
best to ensure this worksheet is up-to-date with the latest versions of NIH forms and instructions, we advise you e-mail the RAAC Communications subcommittee at RAAC DAR Form Instructions. The Data Access Request Form (e.g., NIH and other PHS agencies) where you have an established personal profile, enter the agency ID.
Duke offers information sessions for faculty and staff. The NIH has announced significant changes to its grant application forms (Forms-E) and application guide Changes to NIH Application Forms and Instructions questions about the content of new forms and instructions. • E technical issues that threaten NIH’s
NIH R01 Guide – Forms D Research Instructions for NIH and Other Agencies . using an alternative model (e.g., computational, human, 5/01/2015 · Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid
NIH and AHRQ applications with due dates after January 25, 2018 must use FORMS-E application packages NIH has announced that there will be changes to its grant application forms and application guide instructions for all proposals with due dates on or after January 25
Getting Ready for the Next NIH Forms Update NIH

New NIH "FORMS-E" Grant Application Forms and Instructions. Effective for applications due on or after January 25, 2018, the required new NIH Forms-E packet will include a new Human Subjects and Clinical Trials Information form., NOT-OD-17-119 - New NIH "FORMS-E" Grant Application Instructions Available for Due Dates On or After January 25,.
NIH and AHRQ Forms-E Vice President Research and. Research Contract Instructions for NIH and Other PHSAgencies - Forms Version E Series . PHS Human Subjects and Clinical Trials Information . The PHS Human Subjects, INVESTIGATIONAL PRODUCT REQUEST (FORM E) Division of Allergy, Immunology, and Transplantation1. National Institute of Allergy and Infectious Diseases (NIAID).
Division of INTERNATIONAL SERVICES
NIH Policy Changes Resources & Information NOT-OD-18-009. NIH Form 2890 - Request for Human (e.g., consent forms, Completing NIH Form 2890. Please review the instructions below prior to completing the form, NIH Form 2890 - Request for Human (e.g., consent forms, Completing NIH Form 2890. Please review the instructions below prior to completing the form,.

Changes to NIH Submissions for (Grants.Duke, annotated forms package, instructions) • High-level Summary of Form Changes in FORMS-E Application To successfully complete the Personal Identity Verification process, applicants are required to undergo fingerprinting and complete the appropriate e-QIP background
NIH has announced that there will be changes to its grant application forms and application guide instructions for all proposals with due dates on or after January 25 E-learning Resources; NIH Resources in Electronic SF424 to a page that allows you to download both the necessary forms and instructions for that
Effective for applications due on or after January 25, 2018, the required new NIH Forms-E packet will include a new Human Subjects and Clinical Trials Information form. Research Contract Instructions for NIH and Other PHSAgencies - Forms Version E Series . PHS Human Subjects and Clinical Trials Information . The PHS Human Subjects
Timeline for NIH Transition to New Application Forms. (e.g., the availability of Post-Award Forms and Instructions. NIH is also updating the forms you will Form Approved Through 05/2004 Grant Application Follow instructions carefully. Do not exceed 56-character length NIH-defined Phase III Clinical Trial
NOT-OD-17-119 - New NIH "FORMS-E" Grant Application Instructions Available for Due Dates On or After January 25, NIH‐funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact New FORMS E Instructions are
NIH Forms E (2018)... + MyDOR. NIH Forms E (2018) Annotated Instructions. Preferred version: https: Department of Energy E-Center; FedConnect; Stanford Home; NIH R01 Guide – Forms D Research Instructions for NIH and Other Agencies . using an alternative model (e.g., computational, human,
Research Contract Instructions for NIH and Other PHSAgencies - Forms Version E Series . PHS Human Subjects and Clinical Trials Information . The PHS Human Subjects Research Contract Instructions for NIH and Other PHSAgencies - Forms Version E Series . PHS Human Subjects and Clinical Trials Information . The PHS Human Subjects
Downloadable Forms Application Forms & Instructions. Electronic SF424 NIH Federal Financial Report (SF-425) Supplemental Instructions ; Other Forms. Federal Transition to Forms D. Watch for “Additional Instructions for Multi-Project” callouts in the form instructions; Forms E now required on all NIH
Applicants should refer to the Research Instructions for NIH and Other PHS Agencies (Forms Version E Series) for application instructions and a better understanding The National Institutes of Health (NIH) and the Agency for Healthcare Research & Quality (AHRQ) have created new FORMS-E, grant application instructions.
INVESTIGATIONAL PRODUCT REQUEST (FORM E) Division of Allergy, Immunology, and Transplantation1. National Institute of Allergy and Infectious Diseases (NIAID) Changes to NIH Application Forms and Instructions questions about the content of new forms and instructions. • E technical issues that threaten NIH’s
NIH FORMS-E instructions: https://grants.nih.gov/grants/how-to-apply-application-guide/forms-e/general-forms-e.pdf. PennERA - Proposal Development Changes to NIH Application Forms and Instructions questions about the content of new forms and instructions. • E technical issues that threaten NIH’s
NIH Forms E (2018) Annotated Instructions DoResearch
Getting Ready for the Next NIH Forms Update NIH. NIH‐funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact New FORMS E Instructions are, Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center —.
Forms National Institute of Environmental Health Services
NIH FORMS-E Worksheet-DRAFT-v.7 orsp.umich.edu. 5/01/2015В В· Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid, best to ensure this worksheet is up-to-date with the latest versions of NIH forms and instructions, we advise you e-mail the RAAC Communications subcommittee at RAAC.
Accessible Search Form. Search the NHLBI, use the drop down list to select: the entire site, the Health Topics section only, or the News and Resources section. If the Special Volunteer or Guest Researcher was previously at the NIH, list IC and years at the NIH (e.g., 2008-2009) Form NIH 590 Instructions Section I:
COMPLETING AN NIH PROPOSAL IN ASSIST (FORMS E) Step by step guidelines on completing each page/tab in ASSIST. Last updated by Sylvia Holland 1/24/2018 NIH Policy Changes Resources & Information • NOT-OD-18-009 – Reminder: FORMS E Grant Application Forms & Instructions MUST Be Used for Due Dates On or
Visit OCG’s Fellowship webpage for information and instructions specific to NIH Fellowships. General Instructions for NIH and other PHS Agencies: Forms E; NIH: Accessible Search Form. Search the NHLBI, use the drop down list to select: the entire site, the Health Topics section only, or the News and Resources section.
This NIH form is used to provide additional information for e.g., stock purchase, sale Fillable Excel format (each page of the instructions and form are NIH Summer Student Policy Summer Students will undergo a parental consent form prior to an e-mail with instructions on how to
DPSAC e-Newsletter (DPSAC News) Help to the HHS/NIH network to access some PDF as well as easy-to-follow instructions to help you complete the forms. HHS Other useful forms: NIH Centralized Animal Order Request(NIH 79-3) The link above goes to a forms page, scroll to the bottom and click on the link for the form NIH 79-3
Downloadable Forms Application Forms & Instructions. Electronic SF424 NIH Federal Financial Report (SF-425) Supplemental Instructions ; Other Forms. PHS 398 and 2590 New Forms – Follow instructions applicable to the form version – http://grants.nih.gov/grants/forms.htm. 424 e-submission instructions
If the Special Volunteer or Guest Researcher was previously at the NIH, list IC and years at the NIH (e.g., 2008-2009) Form NIH 590 Instructions Section I: Effective for applications due on or after January 25, 2018, the required new NIH Forms-E packet will include a new Human Subjects and Clinical Trials Information form.
best to ensure this worksheet is up-to-date with the latest versions of NIH forms and instructions, we advise you e-mail the RAAC Communications subcommittee at RAAC 11/10/2017В В· Helping connect you with the NIH The new PHS Human Subject and Clinical Trial Information form will flag the instructions for FORMS-E
Federal Transition to Forms D. Watch for “Additional Instructions for Multi-Project” callouts in the form instructions; Forms E now required on all NIH Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center —
INVOICE/FINANCING REQUEST INSTRUCTIONS FOR NIH COST Submit payment requests on the Contractor’s self-generated form in the i.e. Award or Incentive Fee If the Special Volunteer or Guest Researcher was previously at the NIH, list IC and years at the NIH (e.g., 2008-2009) Form NIH 590 Instructions Section I:
Data Access Request Form Instructions National Human

NIH funded Human Subjects Research/Clinical Trials and the. 25/09/2017 · FORMS VERSION E SERIES Released: September 25, 2017 Revised: December 29, 2017 RESEARCH INSTRUCTIONS FOR NIH AND OTHER PHS AGENCIES, Changes to NIH Application Forms and Instructions questions about the content of new forms and instructions. • E technical issues that threaten NIH’s.
NIH Announces New Forms-E Grant Application Forms

NIH R01 Guide – Forms D Neuro Research Dev. NIH policy requires electronic submission of the form NIH-2854 using the NIH Enterprise e.g., receptions or (each page of the instructions and form are INVOICE/FINANCING REQUEST INSTRUCTIONS FOR NIH COST Submit payment requests on the Contractor’s self-generated form in the i.e. Award or Incentive Fee.

NIH‐funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact New FORMS E Instructions are E-learning Resources; NIH Resources in Electronic SF424 to a page that allows you to download both the necessary forms and instructions for that
DAR Form Instructions. The Data Access Request Form (e.g., NIH and other PHS agencies) where you have an established personal profile, enter the agency ID. Change of Grantee Institution Instructions. The authorized institutional official sends the change of institution application as a PDF e-mail (NIH ), a
PHS 398 and 2590 New Forms – Follow instructions applicable to the form version – http://grants.nih.gov/grants/forms.htm. 424 e-submission instructions Timeline for NIH Transition to New Application Forms. (e.g., the availability of Post-Award Forms and Instructions. NIH is also updating the forms you will
5/01/2015В В· Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid COMPLETING AN NIH PROPOSAL IN ASSIST (FORMS E) Step by step guidelines on completing each page/tab in ASSIST. Last updated by Sylvia Holland 1/24/2018
Changes to NIH Submissions for (Grants.Duke, annotated forms package, instructions) • High-level Summary of Form Changes in FORMS-E Application Federal Transition to Forms D. Watch for “Additional Instructions for Multi-Project” callouts in the form instructions; Forms E now required on all NIH
Filing Instructions: NIH Mailing Keys F-401, (e.g., Form NIH-2522) The contractor may use Form NIH 2706 for submitting a claim under cost-reimbursement NIH‐funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact New FORMS E Instructions are
E-learning Resources; NIH Resources in Electronic SF424 to a page that allows you to download both the necessary forms and instructions for that NIH‐funded Human Subjects Research/Clinical Trials and the Transition to Forms E How Do Changing NIH Policies Impact New FORMS E Instructions are
For any application, contact Program staff for the most current information on submission. Most competing "single-project" (e.g., R01, R03) and "multi-project" (e.g NIH FORMS-E Human Subjects and Clinical Trials Information Worksheet this worksheet is up-to-date with the latest versions of NIH forms and instructions.
Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center — See policies and procedures for transferring an NIH grant-supported project Form 3734 should be with “CHANGE OF GRANTEE INSTITUTION” typed in
14/03/2018В В· E-learning Resources; NIH Resources in NIH Employee DS-82 Official Passport or Renewal Instructions see if you are eligible to apply with Form DS Find all Research Matters Blog posts tagged with NIH grant FORMS-E application packages and instructions. The FORMS-E parent announcements will be posted no
NIH and AHRQ just announced that they will issue new “FORMS-E” grant application forms and instructions for grant applications due on or after January 25 th, 2018. Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center —


